Since Sept. 20 1997
Hidekazu Ido
Wellchemie Laboratory
wellchemlab(atmark)yahoo.co.jp

Public technical report 2021-500018
The
periodic
table of elements is the basic table in science. It has evolved since
Russian chemist Dmitri Mendeleev published the prototype of periodic
table in 1869. The long
periodic table is useful and most popular in various periodic tables1.
It is a table in which the elements are arranged in order of atomic
number, which well represents the chemical properties based on the
electron configuration of the atoms (figure 1).
However
it
may not be able to fully express the similarity of chemical
properties, the arrangement of main group elements and transition
elements, and the stability of nuclei.
Here
we show that the element table with a fractal structure greatly
improves the above problems and expands the function of the periodic
table. In the periodic table, the elements are arranged mainly
focusing on the seven rows (periods) corresponding to the periodicity
of chemical properties. That is, the elements are arranged by focusing
mainly on the seven rows called the period. On the other hand, in the
fractal table of elements, we focused on the fact that the group of
elements has similar chemical properties, and clarified a new
relationship between the elements and nuclei by forming a fractal
structure with the group as the basic unit.
We
expect
that the fractal table of elements contributes the development of
chemistry, physics, biology and other sciences.
In
the long periodic table, the elements are arranged in order of atomic
number in periodic units, and elements with similar chemical properties
are arranged vertically in the table. It is very useful, but there are
some problems. The elements are classified in the order of s, f, d, p
blocks from the left of the table, which is different from the order of
azimuthal quantum number. Since the table is arranged in periodic units,
groups 1 and 18 are separated, and there is no continuity. Main group
elements are divided into group 1 〜 2 and group 13 〜
18 by transition elements. Since the positions of main group elements
and transition elements with similar valences (for example, group 5 and
15 and group 7 and 17) are separated, their similarity is not expressed.
Lanthanoids and actinoides are listed in a separate table. The shell
model of atomic nucleus is not considered, and the magic numbers (2, 8,
40, 50, 82, 114) do not appear in the elemental arrangement.
Therefore,
we studied the structure of the element table that can express chemical
similarities without division. Approximately fractal figures are found
in every aspect of nature and seem to be related to the essence of
nature, so we decided to apply the fractal structure to the element
table.
A
group with similar chemical properties was defined as a basic structure
(cell) consisting of 9 squares. Figure 2 shows the cell in the fractal
element table. In the cell, the elements of each group are arranged in
the order of the numbers listed in the cells from the one with the
smallest atomic number. The cells were then arranged to form a fractal
structure to create a fractal element table.
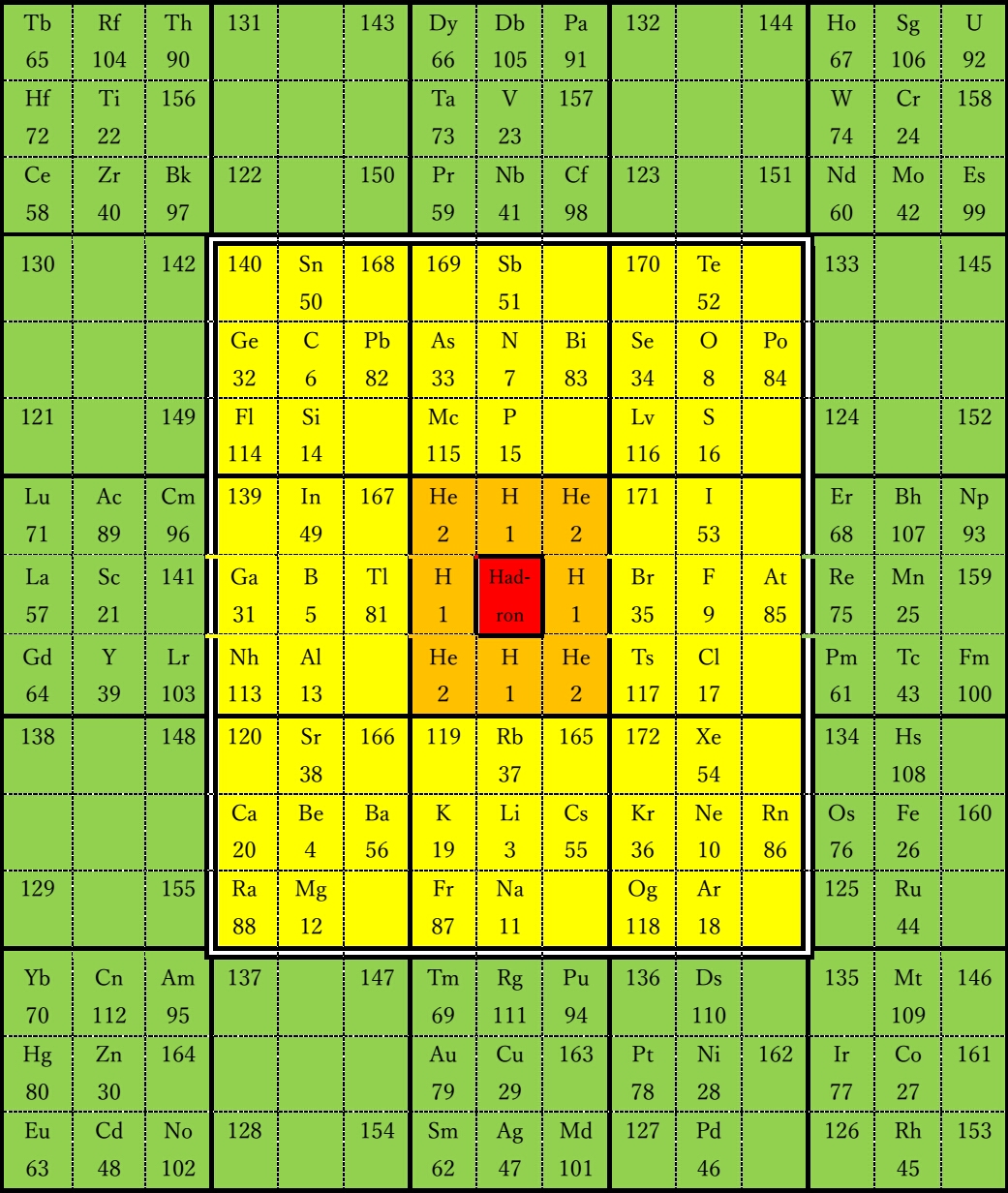
Figure
3 shows a fractal table of elements. It consists of the 0th to 3rd
layers. The 0th layer (central part) is hadron, the 1st layer is an
element close to hadron, the 2nd layer is s, p block element, main group
element, and the 3rd layer (peripheral part) is d, f, g block element,
transition element. Hydrogen and helium in the 1st layer are the
simplest and closest elements to hadrons with the fewest electrons.
Hydrogen symbolizes odd-numbered electrons and helium symbolizes
even-numbered electrons. Main group elements and transition elements
with similar valences are close to each other.
Main group elements are gathered in the 1st and 2nd layers
without being separated by transition elements. Since the alkali metal
cell and the rare gas cell are in contact with each other, each period
is continuous. Lanthanoids and actinoids are listed in the same table as
other elements.
In
Figure 4, the elements whose stable isotopes with the highest abundance
ratio have magic numbers are shown in yellow, and artificial radioactive
elements other than transuranium elements are shown in blue. The
elements whose stable isotopes with the highest abundance ratio have
magic numbers (2, 8, 40, 50, 82, 114) are lined up parallel to the
diagonal line connecting the lower left and upper right of the table.
Artificial radioactive elements other than transuranium elements are
concentrated in the middle right part of the table. There are elements
with atomic numbers 126, 146, 160, and 162 in the vicinity of the line
passing through Ni parallel to the diagonal line connecting the lower
left and upper right of the table, and these numbers may be magic
numbers.
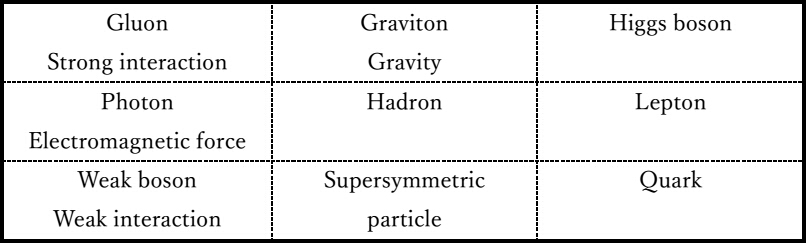
Figure
5 is a table in which elementary particles related to hadrons located in
the center (0th layer) of the fractal table of elements are arranged in
cells. The left and right columns of the cell are the elementary
particles mentioned in the Standard Model2. The particles located opposite each other in the
cell are related to each other. Hadrons are particles in which quarks
are bound by a strong interaction. Neutrons are transformed into protons
by a weak interaction. Atomic nuclei consisting of hadrons and electrons
form atoms by electromagnetic interaction. Graviton and supersymmetric
particles are elementary particles mentioned in superstring theory3.
The
Fractal Table of Elements reflects both the chemical properties of the
elements due to the electron configuration and the properties of the
nuclei due to the nuclear shell model.
It
is expected to contribute to developing of chemistry, physics, biology
and other sciences.
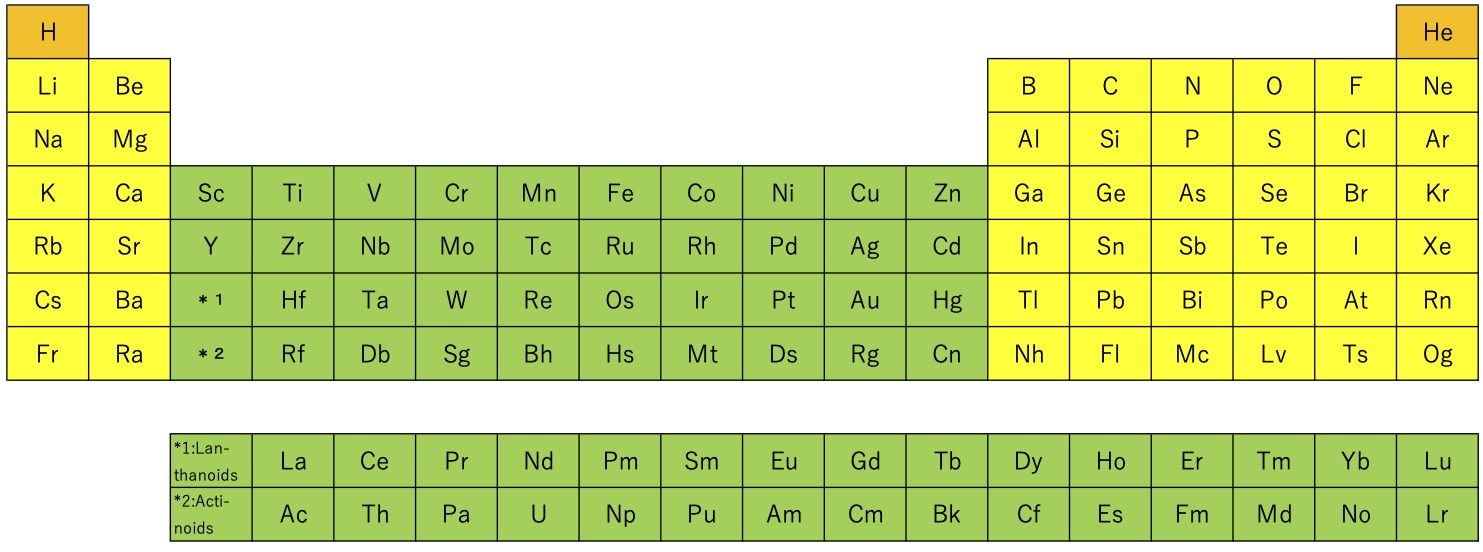
Figure 1 Long periodic table of elements.
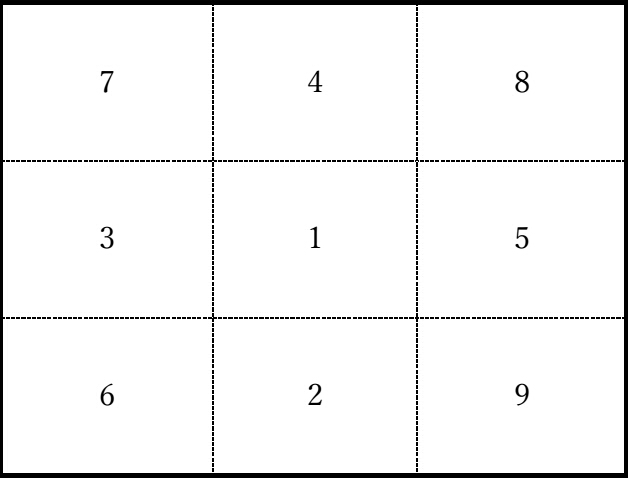
Figure 2 The cell in the fractal table of elements.

Figure
3 The fractal table of elements
red:
0th layer, orange: 1st layer, yellow: 2nd layer, green: 3rd layer.
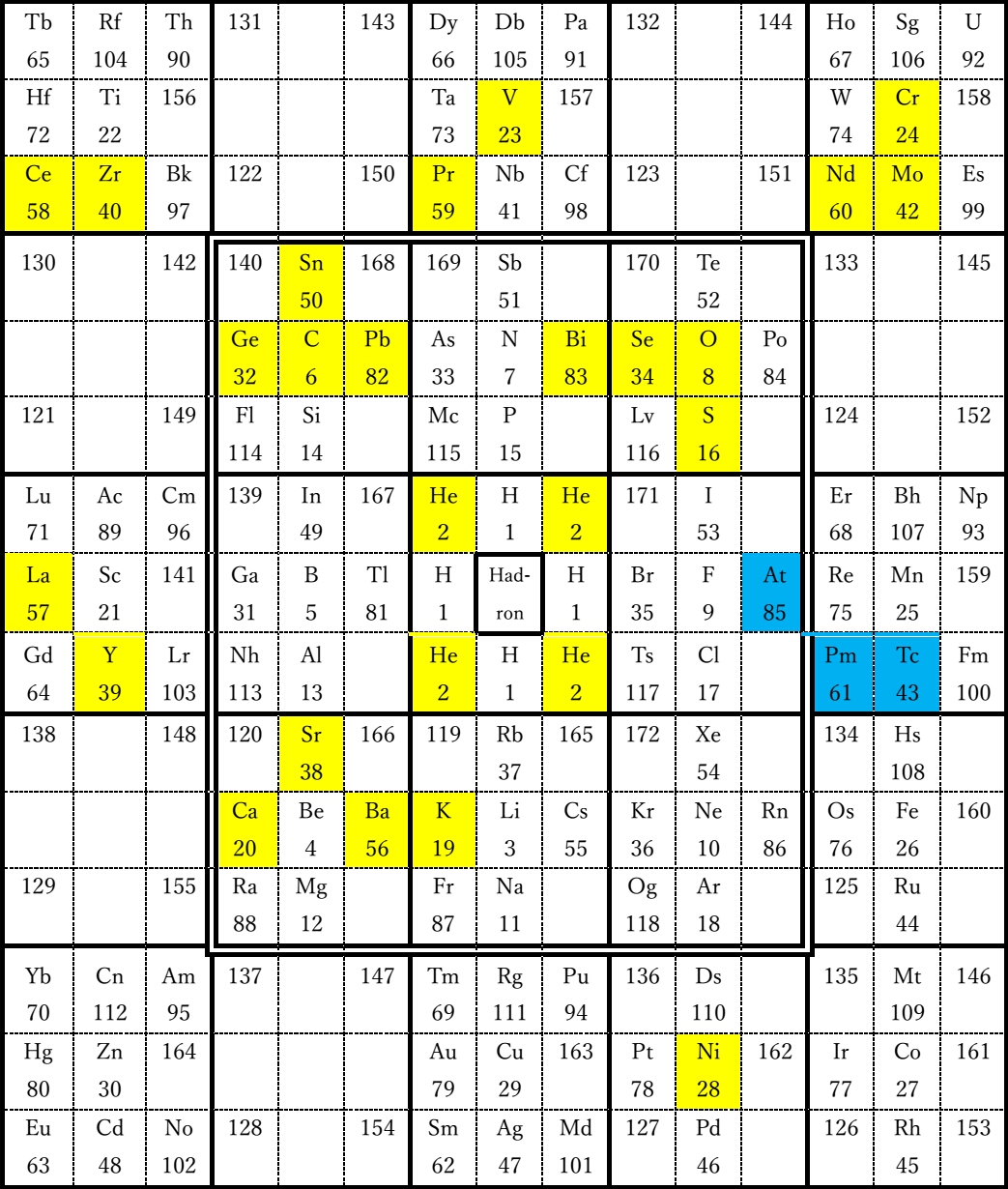
Figure
4 The elements whose stable isotopes with the highest abundance ratio
have magic numbers are shown in yellow, and artificial radioactive
elements other than transuranium elements are shown in blue.
1. Marchese, F. T. The Chemical Table: An Open Dialog
between Visualization and Design. 2008 12th International Conference
Information Visualisation, London, 75-81. (2008).
2. Beringer et al. (Particle Data Group), Review of Particle Physics. Phys.
Rev. D 86, 010001 (2012).
Hidekazu Ido
wellchemlab(atmark)yahoo.co.jp